29
2022
-
08
Effects of early intervention of Clostridium butyricum on intestinal flora and short chain fatty acids in broilers
Reading:This experiment was conducted to study the effects of early intervention of Clostridium butyricum (Clostridium butyricum) on intestinal short-chain fatty acids (SFAs) and flora structure of Enterotoxigenic Escherichia coli (ETEC) in broilers. 200 1-day-old ROSS broilers were selected and divided into two groups, 100 in each group. Three days before the experiment, one group was fed with 1mL of Clostridium butyricum with a concentration of 109 CFU/mL every day, and the other group was fed with 1mL of normal saline. At 7 days of age, broilers fed with saline were divided into control group (Control) and E. coli group (EC). Broilers fed with Clostridium butyricum were divided into Clostridium butyricum group (CB) and Clostridium butyricum E. coli group (CB EC). From the age of 7 days to the end of the experiment, EC group and CB EC group were fed with 1mL(108 CFU/mL) enterotoxigenic E. coli solution for challenge, while Control group and CB group were fed with 1mL normal saline. Slaughter sampling at 3d and 7d after ETEC challenge. The results showed that: ① In the caecum of broilers, at the age of 10 days, compared with the Control group, the acetic acid content in CB group was significantly increased (P<0.01), and the acetic acid content in EC group was significantly decreased (P<0.05). The content of butyric acid in CB group and CB EC group is relatively similar, and is significantly higher than that in Control group and EC group (P<0.01). At 14 days of age, compared with Control group, the contents of acetic acid, propionic acid, butyric acid and isobutyric acid in CB group were significantly increased (P<0.05), while those in EC group were significantly decreased (P<0.05). ②At the age of 10 days, in the CB group, the relative abundance of rumen cocci was the highest, which was significantly higher than that of the other experimental groups (P<0.05). It can be seen that the early intervention of Clostridium butyricum in broilers can promote the production of intestinal short-chain fatty acids and improve the structure of cecal flora to a certain extent.The full text has been published in the 11th issue of Feed Industry in 2021.
Enterotoxigenic Escherichia coli (Enterotoxigenic Escherichia coli,ETEC) can easily cause diarrhea, intestinal diseases and even death of primary livestock and poultry, which has been a major factor restricting the healthy and efficient breeding of livestock and poultry. In the past, antibiotics were commonly used to treat or control the negative effects caused by E. coli. In recent years, with the increasingly prominent problem of antibiotic resistance and residue, the use of antibiotics in the livestock and poultry industry has been restricted worldwide. Clostridium butyricum is an important member of probiotics. Recent studies have shown that Clostridium butyricum has significant anti-inflammatory, regulating intestinal flora, enhancing immunity and other biological functions [1-5]. In addition, Clostridium butyricum can also promote the production of short chain fatty acids, lactic acid and digestive enzymes, and improve the health of livestock and poultry by regulating intestinal flora and promoting the secretion of immune defense factors [6]. Yang et al. [7] showed that the addition of Clostridium butyricum in the diet could reduce the number of Escherichia coli in the caecum of broilers and increase the number of Bifidobacterium and Lactobacillus in the caecum of broilers. Shen Lulu et al [8] showed that early intervention of Clostridium butyricum could reduce the expression of intestinal inflammatory factors and antimicrobial peptide genes in E. coli-attacked broilers, and reduce the diarrhea of chicks. Zhang et al. [2] showed that dietary Clostridium butyricum could improve the growth performance, intestinal barrier function and digestive enzyme activity of E. coli-infected broilers. However, the effect of early intervention of Clostridium butyricum on the intestinal short-chain fatty acid content of broilers infected with E. coli has not been reported, and there are few studies on early intervention on intestinal flora. Therefore, this experiment studied the changes of intestinal short-chain fatty acid content and flora structure of Clostridium butyricum in broilers after E. coli infection, in order to provide a scientific basis for the prevention and control of E. coli in broilers and even poultry production.
1. Materials and Methods
1.1 Test Animals and Species
The 1-day-old broilers were ROSS 308 broilers, which were purchased from Tengda Incubation Plant in Zhenjiang, Jiangsu Province. Test Clostridium butyricum (No. MIYAIRI II 588) was purchased from Miyarisan Corporation, Japan. Enterotoxigenic Escherichia coli K88 (number: CVCC1556) was purchased from China Veterinary Microbial Stra Preservation Management Center.
1.2 Test Design
According to the principle of no difference in body weight, 200 ROSS308 broilers in good health were randomly divided into 2 groups, 100 chickens in each group, namely control group (Control group) and Clostridium butyricum group (CB group). 1-3 days after the start of the test, the chickens in the Control group were fed with 1mL of normal saline every day, and the chickens in the CB group were fed with 1mL of Clostridium butyricum solution with a concentration of 109 CFU/mL. At the 7th day, the CB group was randomly divided into Clostridium butyricum group (CB group) and Clostridium butyricum Escherichia coli group (CB EC group), at the same time, the Control group was randomly divided into control group (Control group) and E. coli group (EC group), with 50 chickens in each group. EC group and CB EC group were fed with 1mL of E. coli bacterial solution with a concentration of 108 CFU/mL every day from 7 days of age to the end of the test, while Control group and CB group were fed with 1mL of normal saline every day from 7 days of age to the end of the test for 14 days. The experimental design and grouping are shown in Table 1.

1.3 feeding management
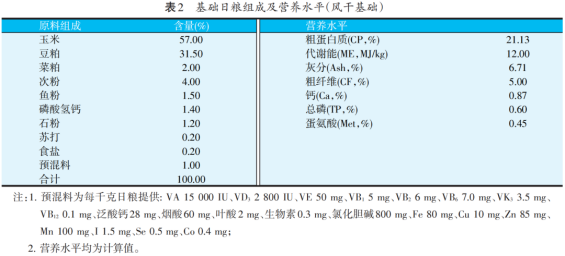
The test site is the animal test base of Zhejiang Academy of Agricultural Sciences. Before the test, the animal house and feeding equipment were inspected and strictly disinfected. The temperature in the animal room was adjusted by incandescent lamp. The temperature was kept at 37 ℃ in the first week, and then gradually decreased by 2~3 ℃ every week until it was consistent with the room temperature and kept for 24 hours. The experimental broilers were fed free to feed and drink water, fed manually, three times a day. The composition of basal diets and their nutrient levels are shown in Table 2.
1.4 sample collection and preservation
At the age of 10 and 14 days, 8 broilers were randomly selected from each group for slaughter. The contents of ileum and cecum were collected in 5mL centrifuge tubes, stored in liquid nitrogen, and stored in an ultra-low temperature refrigerator at -80 ℃ for bacterial DNA extraction.
Determination of short chain fatty acids in 1.5
Weigh 0.1g of cecal contents in a centrifuge tube, add 9 times the volume (mL) of PBS solution, mix evenly, centrifuge at 12 000 r/min for 10 min, take 500 μL of supernatant, add 100 μL of 25% (mass volume ratio) crotonic acid metaphosphate mixture, vortex and mix evenly, and store overnight in a refrigerator at -20 ℃ for 24 h. Before loading the machine, centrifuge at 12 000 r/min for 10 min, take the supernatant, filter it on a 0.22 μm filter membrane, take 150μL on the sample bottle, and measure SCFAs content on a gas chromatograph. The chromatographic conditions were as follows: column temperature 70 ℃; FID1 detector was used; detection temperature 250 ℃; carrier gas was nitrogen; pressure 37.5 kPa; oxygen pressure 50 kPa, hydrogen 50 kPa, sensitivity 10-1, attenuation 3.0.
1.6 Fecal DNA Extraction and High-Throughput Sequencing Analysis
Fecal DNA was extracted according to the procedure of QIAamp Fast DNA Stool Mini Kit, QIAGEN, Germany. The V3 ~ V4 region of bacterial 16SrRNA gene was amplified by PCR using the extracted DNA as template and primers of 338F(5 ′ ACTCCTACGGGAGGCAGCA-3) and 806R (5 ′ GGACTACHVGGGTWTCTAAT-3). Pair end (250 bp × 2) and Hiseq2500 platform were used for PE250 sequencing.
1.7 Data Processing and Statistical Analysis
Uparse software (Uparse V7.0.1001) was used to cluster the effective sequences after quality control into operational classification units (operational taxonomic unit,OTU) according to similarity ≥ 97%. The QIIME(Version 1.7.0) default parameters were used to calculate the alpha diversity index (including the Ace and Chao analysis of the flora abundance index, the Shannon index Shannon and Simpson index Simpson analysis of the flora diversity index, and the Good coverage analysis of the coverage index) and species distribution of each sample. In order to compare the differences in the structure of the bacterial community at different times using BPW, the FactoMineR software package in the R software (Version 2.15.3) was used to perform principal coordinate analysis (PCoA) on the similarity of the bacterial community structure between samples. The results of the relative abundance of short-chain fatty acids and intestinal flora were analyzed by SPSS, and SPSS was 18.0 to analyze the significance of the experimental data. The results are expressed as "mean standard error (Mean ± SEM)", with P<0.05 indicating a significant difference and P<0.01 indicating a very significant difference.
2. Results and analysis
Content of short chain fatty acids in 2.1 ileum and cecum
From Table 3, it can be seen that in the ileum of broilers at the age of 10 days (3 days after the attack) and 14 days (7 days after the attack), the content of short chain fatty acids is very small, and the difference between the groups is not significant (P>0.05).
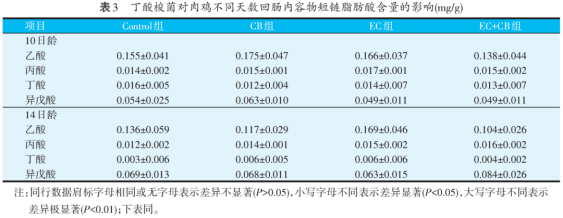
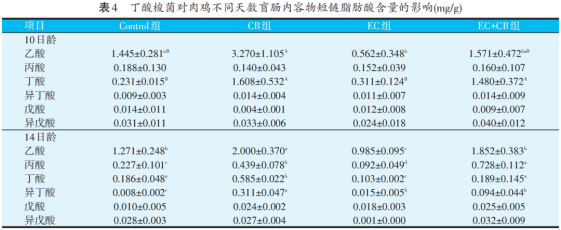
As can be seen from table 4, in the caecum of broilers, at the age of 10 days, compared with the Control group, the acetic acid content in CB group was significantly increased (P<0.01), and the acetic acid content in EC group was significantly decreased (P<0.05). The content of butyric acid in CB group and CB EC group is relatively similar, which is significantly higher than that in Control group and EC group (P<0.01). At 14 days of age, compared with Control group, the contents of acetic acid, propionic acid, butyric acid and isobutyric acid in CB group were significantly increased (P<0.05), while those in EC group were significantly decreased (P<0.05).
High-throughput sequencing of 2.2 cecal content bacteria
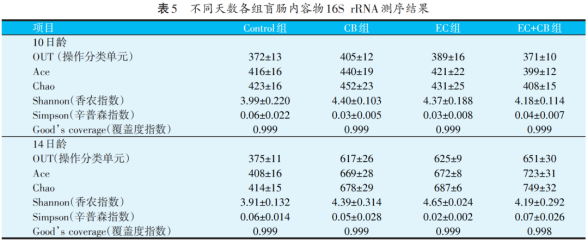
High-throughput sequencing technology was used to determine the cecal flora structure of broilers aged 10 and 14 days. The results are shown in Table 5. Broilers aged 10 and 14 days obtained 371~405 and 375~651 OUT on average respectively, and the coverage index (good coverage) of each sample was above 0.998. The results show that almost all the sequences in the samples have been detected. And by making a dilution graph observation, as shown in Figure 1, the sequencing curve of all samples tends to be flat, which shows that the sequencing results are reasonable, and the sequencing depth is 63 390 times.
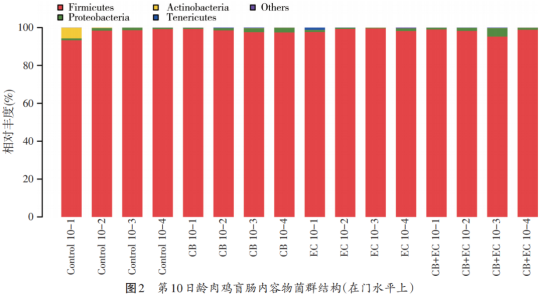
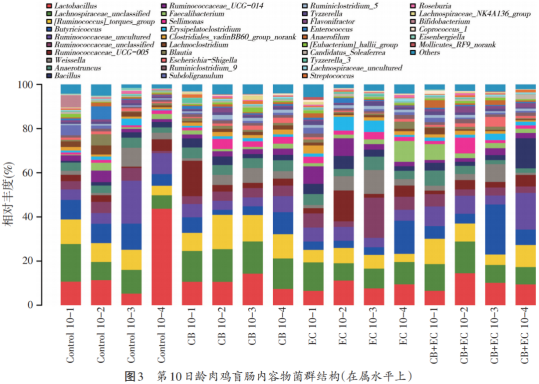
As shown in fig. 2, at the 10th day of broiler cecum, the thick-walled bacteria (Fimicutes) were the main species in each group, with the relative abundance accounting for more than 90%, followed by the proteobacteria (Proteobacteria), actinomycetes (Actinobacteria) and the soft-walled bacteria (Tenericutes). Figure 3 shows the relative abundance of caecum in 10-day-old broilers. The main flora in each group are lactic acid bacteria, trichotheca, rumen coccus, butyric acid coccus, Weiss bacteria (Weissella), Bacillus (Bacillus) and so on.
At the age of 14 days, the flora structure of each group changed. At the phyla level (see Figure 4), the phyla, proteobacteria, bacteroides (Bacteroidetes), actinomycetes and cyanobacteria (Cynaobacteria) were the main species in each group. At the genus level, from the proportion of each genus shown in Figure 5, it can be seen that lactic acid bacteria, rumen coccus, hair snail family, rumen coccus family, soft clostridium (Faecalibacterium), butyric acid coccus, bacillus, etc. are the main bacteria.
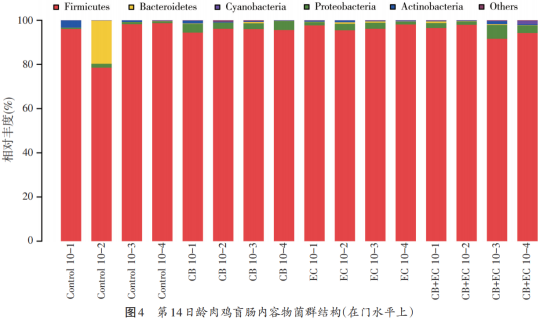
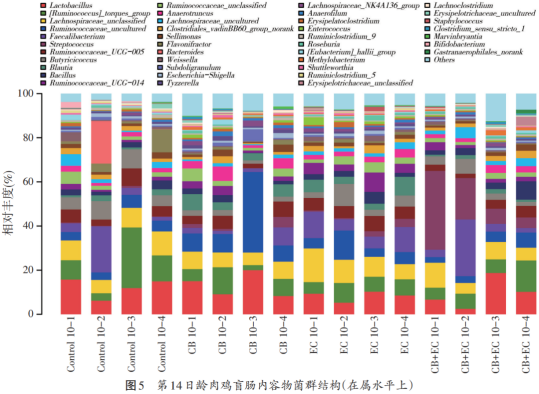
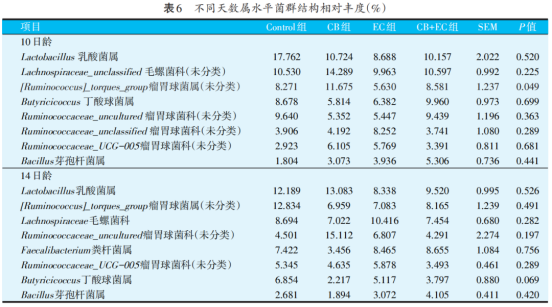
Table 6 shows the relative abundance of the top eight bacteria in each group at the genus level at the age of 10 and 14 days of broiler chickens, and the difference between the bacteria with greater relative abundance in each group was not significant (P>0.05). At the age of 10 days, there were relatively more lactic acid bacteria, trichotheca, butyricum and rumen coccus, and the relative abundance of rumen coccus in CB group was significantly higher than that in other groups (P<0.05). At 14 days of age, the relative abundance of the four genera were lactic acid bacteria, trichotheca, rumen coccus and rumen coccus, but the differences were not significant (P>0.05).
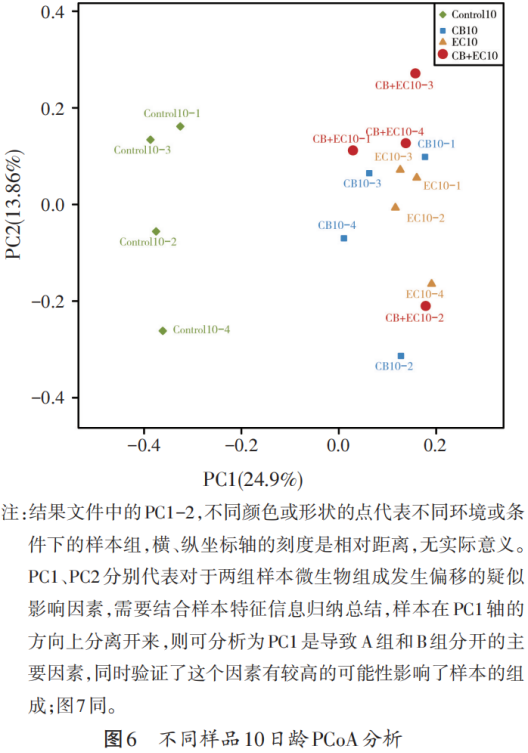
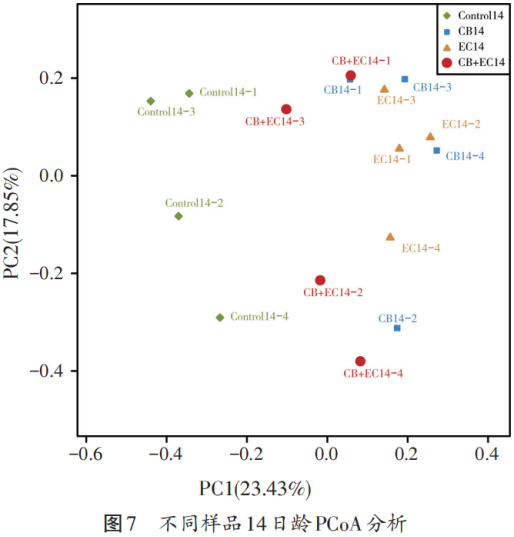
Figures 6 and 7 are principal coordinate analysis (PCoA) plots, using this method, the similarity or difference in the relative abundance of each group of samples can be observed. It can be seen from Figure 6 that the similarity of the Control group at the age of 10 days of broilers is quite different from that of the other test groups. At the same time, it can be seen from Figure 7 that the Control group, CB EC group and the other two groups are less similar at 14 days of age of broilers.
3. Discussion
Effects of Clostridium Butyricum 3.1 on Intestinal Short-chain Fatty Acid Content in Broilers
The intestinal tract is the largest immune organ of the animal body, and it is also the main place for the digestion and absorption of nutrients. The intestinal health largely determines the growth performance and overall health of animals. Short-chain fatty acids are the main products of microbial metabolism, also known as volatile fatty acids, which are mainly composed of acetic acid, propionic acid, butyric acid, valeric acid, and isovaleric acid with carbon atoms less than 6 in the carbon chain [9]. Studies have shown that short-chain fatty acids have important roles in energy supply, anti-inflammatory, immune regulation, maintaining intestinal homeostasis and improving intestinal flora [10-12]. The short-chain fatty acid butyric acid produced by Clostridium butyricum through the fermentation of fibers in the intestine is the main energy substance of the body's colonic epithelial cells, and through oxidation for the cell energy supply, enhance the intestinal immune and barrier function. The metabolites of butyric acid can improve the intestinal digestion and absorption of nutrients in feed, and supplement the nutrients needed by the body [13-14]. Guo Chuanzhen et al. [15] added different doses of sodium butyrate in the diet to increase the concentration of short-chain fatty acids in the intestinal tract of broilers. This is similar to this test. In this test, the currently prepared culture solution containing viable Clostridium butyricum was directly used, and the fermentation and proliferation continued in the anaerobic environment of the intestinal tract to produce butyric acid. The test results showed that feeding Clostridium butyricum increased the content of acetic acid, butyric acid and isobutyric acid in the cecum, and with the delay of feeding time, the short-chain fatty acid in the cecum increased trend. In addition, E. coli can reduce the content of acetic acid, which suggests that Clostridium butyricum can inhibit E. coli to reduce cecal short chain fatty acid content to a certain extent.
Effects of Clostridium Butyricum 3.2 on Intestinal Flora Structure of Broilers
Clostridium butyricum is an important class of probiotics, which can promote the growth of intestinal beneficial bacteria and inhibit the proliferation of harmful bacteria [16]. Intestinal flora provides additional metabolic functions for animal hosts, including digestion and absorption of nutrients, fermentation of dietary fiber and synthesis of certain microorganisms. Intestinal flora is closely related to the growth and development of poultry, and is involved in intestinal immune regulation [17]. Research by Liu Tingting et al. [18] has shown that Clostridium butyricum promotes the proliferation of probiotics such as Lactobacillus and Bifidobacterium, and inhibits the growth of Escherichia coli. Yang et al. [7] showed that Clostridium butyricum is beneficial to the balance of intestinal flora in broilers. Zhang[1] and others reported that Clostridium butyricum reduced the number of Escherichia coli and Clostridium perfringens in caecum of broiler chickens attacked by Escherichia coli K88, increased the number of bifidobacteria and improved the intestinal microflora. Huang et al. [19] showed that dietary supplementation of Clostridium butyricum reduced the abundance of Clostridium perfringens in the intestinal tract of northeast chickens with necrotic enteritis. In addition, multiple studies have reported that Clostridium butyricum can regulate the balance of intestinal flora [20-23]. In this experiment, it was found by high-throughput sequencing of bacteria that the caeca of broilers was dominated by the phyla of thick-walled bacteria, the phyla of variant bacteria and the phyla of Bacteroides, which was consistent with the results of previous studies [24]. At the genus level, lactic acid bacteria, trichoderma, rumen coccus, rumen coccus, butyric acid coccus were the dominant bacteria in each group, and the relative abundance of rumen coccus increased significantly in the broiler test group given Clostridium butyricum, while the relative abundance of lactic acid bacteria remained at a high level. Studies have shown that rumen coccus can produce carbohydrate degrading enzymes, of which rumen coccus can secrete a large number of cellulase and hemicellulase, mixed fermentation to produce acetic acid; a number of bacteria in the family of Trichoderma can ferment fiber metabolism to produce butyric acid [25-26]. In order to provide a higher concentration of acid environment for the intestinal tract, reduce intestinal pH, improve intestinal structure. The results suggested that the early intervention of Clostridium butyricum could improve the intestinal flora structure of E. coli-contaminated broilers.
4. Conclusions
In summary, the early intervention of Clostridium butyricum and E. coli attack in broilers can improve the content of short-chain fatty acids such as acetic acid, propionic acid and butyric acid in the caecum of broilers. At the same time, it can inhibit the proliferation of Escherichia coli in the intestinal tract of broilers to a certain extent, and increase the number of lactic acid bacteria and other beneficial bacteria, so as to alleviate the negative impact of E. coli attack. This provides a theoretical basis for the application of Clostridium butyricum as a new feed additive in the actual production of broilers.
References and more can be found in:
Feed Industry, 2021,42(11):44-52
Related News



